Sulfodyne® 5%

Download the document
Sulfodyne® 5%
Sulfodyne - The only stabilised and active form of sulforaphane on the nutraceutical market
The guarantee to get the real sulforaphane from broccoli, and nothing less..Bioavailable sulforaphane, prefer the active form rather than its precursor.Sulforaphane is an organosulfur compound found in crucifers, such as broccoli, Brussels sprouts or cauliflower. It is formed when its precursor, glucoraphanin, is hydrolyzed by an enzyme called myrosinase..Its benefits are based on 3 mechanisms of action:- Antioxidant activity
- Anti-inflammatory activity
- Cellular protection (sulforaphane is a so-called epigenetic molecule)
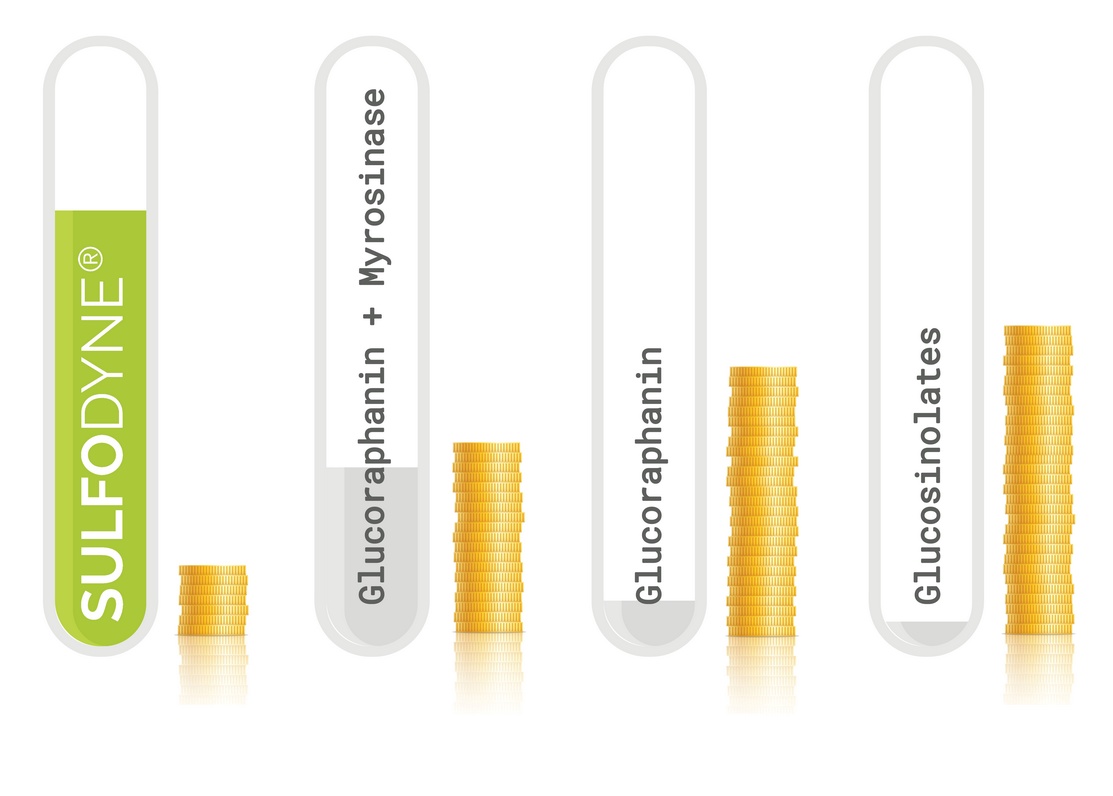
- 10mg of Glucoraphanin can only provide a maximum of 4.06mg of Sulforaphane (by chemical conversion).
- 10mg of ingested GFN leads to 0.45mg on average of SFN in the body (0.1 to almost 1mg, depending on the individual)
- 10mg of GFN with external myrosinase – in laboratory test - provides 2.15mg of SFN (optimal condition), so not everything is transformed (max. 4.06mg by chemical conversion)
- 10mg of GFN with external intake of myrosinase, ingested, provides 1.22mg of SFN in the body (about 1 to 1.48mg) – myrosinase actually improves the metabolism of GFN into SFN
- 10mg of ingested Sulforaphane (Sulfodyne®) provides 7.1mg of Sulforaphane in the body.
- Detoxification : eliminates xenobiotics from the body, and induces the process of detoxification and elimination
- Anti-inflammatory & Joint health : inhibits markers of inflammation and reduces cartilage degradation
- Immunity : increases the activity of NK cells and phagocytosis of Macrophages
- Glucose regulation : allows the activation of the expression of Nrf2, and associated mechanisms
- Women & Men health : exerts anti-inflammatory activity in inflammatory diseases such as endometriosis (EM), in addition to the chemopreventive activity demonstrated by studies (including 300 studies on prostate)
- Studies report also the use in preventive prophylaxis
- Brain : neuroprotective effect on brain and improving behavior and social responsiveness in autism
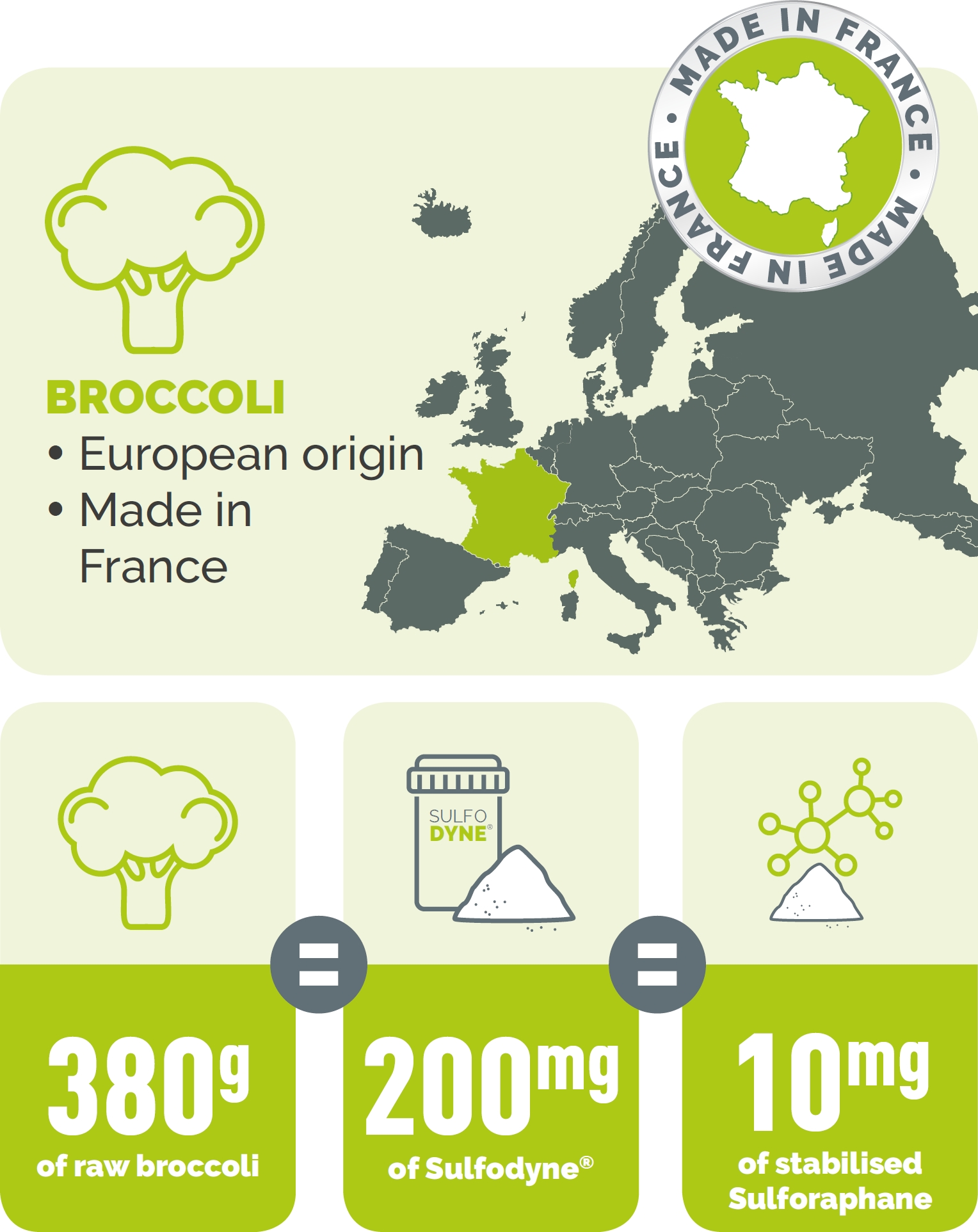
Broccoli - Broccoli : the food of choice
Broccoli is a variety of cabbage native to southern Italy. It is known for its health benefits thanks to its richness in sulfur compounds. Indeed, it is concentrated in glucosinolates, and more particularly in its hydrolysis products among which we find sulforaphane. Broccoli has been shown to be the crucifer with the richest source of sulforaphane, however difficult to extract and stabilize. Thus, many products do not contain sulforaphane, but only its precursor.

Sulfodyne® is the guarantee to get the real sulforaphane, and nothing less. In nutraceuticals, it is important that sulforaphane is provided by a titrated and stable extract (which does not oxidize), without having to be transformed via an endogenous enzyme or supplied. Prefer the active form rather than its precursor ! It's more efficient and less expensive
The only active and natural form of sulforaphane
on the international nutraceuticals market
GUARANTEEING STABILITY AND BIOAVAILABILITY!
Sulforaphane (sometimes sulphoraphane) is a compound within the
|
BIOAVAILABILITY STUDY on Sulfodyne® |
Sulfodyne® is the guarantee to directly get the real sulforaphane, and nothing less.
In nutraceuticals, it is important that sulforaphane is provided by a titrated and stable extract (which does not oxidize), without having to be transformed via an endogenous or added enzyme.
This is an important criteria to ensure the right quantity of sulforaphane in your product.
 |
 |
In addition to the research and studies analysis carried out by the producer of Sulfodyne® - Triballat ingredients - the literature abounds in research and studies demonstrating the unique properties of sulforaphane.
Thus, the site www.sulforaphane.science includes the principales activities listed:
|
Can extend your lifespan Powerfull NRF2 activator Fights inflammation Helps diabetics regulate blood sugar Helpts protect against breast, lung and prostate cancer |
Helps the body eliminate toxins Can reduce symptoms of autism Helps protect against gastric ulcer May slow aging through autophagy Protects a crucial anti-cancer gene (P53) |
Helps maintain a healthy brain Helps prevent cardiovascular disease Helps fight obesity and weight gain Lower bad cholesterol Reduces bladder cancer mortality |
Source : https://www.sulforaphane.science/
-
Studies & Documents(40)
-
News(8)
Sulforaphane in Men with biochemical recurrence after Prostatectomy
Prostate cancer is the most common solid neoplasm in Europe and the second leading cause of cancer mortality in men. Indeed consumption of cruciferous vegetables has been reported to reduce the risks of prostate cancer
Abstract : Increases in serum levels of prostate-specific antigen (PSA) occur commonly in prostate cancer after radical prostatectomy and are designated "biochemical recurrence." Because the phytochemical sulforaphane has been studied extensively as an anticancer agent, we performed a double-blinded, randomized, placebo- controlled multicenter trial with sulforaphane in 78 patients (mean age, 69 ± 6 years) with increasing PSA levels after radical prostatectomy. Treatment comprised daily oral administration of 60 mg of a stabilized free sulforaphane for 6 months (M0–M6) followed by 2 months without treatment (M6–M8). The study was designed to detect a 0.012 log (ng/mL)/month decrease in the log PSA slope in the sulforaphane group from M0 to M6. The primary endpointwas not reached. For secondary endpoints, median log PSA slopes were consistently lower in sulforaphanetreated men. Mean changes in PSA levels between M6 and M0 were significantly lower in the sulforaphane group (+0.099 ± 0.341 ng/mL) than in placebo (+0.620 ± 1.417 ng/mL; P = 0.0433). PSA doubling time was 86%longer in the sulforaphane than in the placebo group (28.9 and 15.5 months, respectively). PSA increases min 20% at M6 were significantly greater in the placebo group (71.8%) than in the sulforaphane group (44.4%); P = 0.0163. Compliance and tolerance were very good. Sulforaphane effects were prominent after 3 months of intervention (M3–M6). After treatment, PSA slopes from M6 to M8 remained the same in the 2 arms. Daily administration of free sulforaphane shows promise in managing biochemical recurrences in prostate cancer after radical prostatectomy
Discussion (...) A stabilized free-form sulphoraphane is a valuable asset in anticancer prevention and therapy. Delivering a predefined dose of sulphoraphane (like any other chemical drug) is mandatory for clinical trials and management. Our future studies will explore its bioavailability in sera of prostate cancer men treated with sulphoraphane. Genomics and proteomics will be performed to have further insight in the mechanisms of action involved. A larger and longer phase III trial is also being designed to confirm its clinical and cost effectiveness.
In conclusion, the effectiveness of a biostable sulphoraphane for decreasing the rate of PSA progression in men with prostate cancer and biochemical recurrence after definitive radical prostatectomy appears promising. The compliance and tolerance were very good. Further studies are required to confirm the clinical importance of this finding.
Effect of Sulforaphane in Men with Biochemical Recurrence after Radical Prostatectomy, Bernard G. Cipolla, Eric Mandron, Jean Marc Lefort,Yves Coadou, Emmanuel Della Negra, Luc Corbel, Ronan Le Scodan, Abdel Rahmene Azzouzi and Nicolas Mottet, Oncologie (2016)
Stabilized Sulforaphane for Clinical Use
Stabilized sulforaphane preparations were as potent as pure SF in inducing the cytoprotective response in cultured cells, and they were more stable and as bioavailable.
Scope: The isothiocyanate sulforaphane (SF) from broccoli, is one of the most potent known inducers of the cytoprotective phase 2 response. Its role in a host of biochemical pathways make it a major component of plant-based protective strategies for enhancing healthspan. Many nutritional supplements are now marketed that purport to contain SF, which in plants exists as a stable precursor, a thioglucoside hydroxysulfate. However, SF in pure form must be stabilized for use in supplements.
Methods and Results: We evaluated the stability and bioavailability of two stabilized SF preparations – an α-cyclodextrin inclusion (SF-αCD), and a SF-rich, commercial nutritional supplement. SF-αCD area-under-the-curve (AUC) peak serum concentrations occurred at 2 hours, but 6 of 10 volunteers complained of mild stomach upset. After topical application it was not effective in up-regulating cytoprotective enzymes in the skin of SKH1 mice whereas pure SF was effective in doing so. Both of these “stabilized” SF preparations were as potent as pure SF in inducing the cytoprotective response in cultured cells, and they were more stable and as bioavailable.
Conclusion: Our studies of a stabilized phytochemical component of foods should encourage further examination of similar products for their utility in chronic disease prevention and therapy.
Stabilized Sulforaphane for Clinical Use: Phytochemical Delivery Efficiency, Jed W. Fahey, Kristina L. Wade, Scott L. Wehage, W. David Holtzclaw, Hua Liu, Paul Talalay, Edward Fuchs, and Katherine K. Stephenson, Molecular Nutrition / Food Research, Nov-2016
Sulforaphane chez les hommes avec récidive biologique après prostatectomie
Le cancer de la prostate est la plus fréquente des tumeurs solides rencontrées en Europe et la seconde cause de mortalité provoquée par le cancer chez l’homme. la consommation de crucifères semble réduire le risque de cancer de la prostate de 40 % [3,4], de son extension de plus de 40 %, et de sa progression de 59% [7], chez les hommes présentant une maladie non métastatique
Résumé L’élévation progressive du taux sérique d’antigène spécifique prostatique (PSA) survenant en cas de cancer de la prostate après prostatectomie totale est désignée comme une récidive biologique. Étant donné que le sulforaphane, substance naturelle, a été beaucoup étudié en tant qu’agent anticancéreux, nous avons effectué une étude en double insu, randomisée, multicentrique, contre placebo, en administrant du sulforaphane à 78 patients (âge moyen : 69 ± 6 ans) présentant une élévation du taux de PSA après prostatectomie totale. Le traitement comprenait une administration orale quotidienne de 60 mg de sulforaphane libre stabilisé pendant six mois (M0–M6) suivie de deux mois sans traitement (M6–M8). L’étude a été conçue pour détecter une diminution de 0,012 log (ng/ml)/mois de la pente logarithmique du PSA dans le groupe sulforaphane de M0 à M6. Ce critère d’évaluation primaire n’a pas été atteint. Concernant les critères d’évaluation secondaires, les courbes logarithmiques médianes de PSA étaient constamment inférieures chez les hommes traités par sulforaphane. Les variations moyennes du taux de PSA entre M6 et M0 étaient inférieures dans le groupe sulforaphane (+0,099 ± 0,341 ng/ml) par rapport au groupe placebo (+0,620 ± 1,417 ng/ml ; p = 0,0433). Le temps de doublement du PSA était 86 % plus long dans le groupe sulforaphane par rapport au groupe placebo (respectivement 28,9 et 15,5 mois). Les augmentations de PSA supérieures à 20 % à M6 étaient significativement supérieures dans le groupe placebo (71,8 %) par rapport au groupe sulforaphane (44,4 %) ; p = 0,0163. La compliance et la tolérance au traitement étaient très bonnes. Les effets du sulforaphane étaient remarquables dès trois mois de traitement (M3–M6). Après traitement, l’évolution des courbes du PSA de M6 à M8 est comparable dans les deux bras. Une administration quotidienne de sulforaphane libre semble prometteuse dans la prise en charge des récidives biologiques du cancer de la prostate après prostatectomie totale.
Conclusion : L’efficacité d’une forme stabilisée de sulforaphane, pour diminuer la progression du taux de PSA chez les hommes présentant un cancer de la prostate et une récidive biologique après une prostatectomie radicale, semble prometteuse. La compliance et la tolérance ont été très bonnes. D’autres études sont requises pour confirmer l’importance clinique de cette découverte.
Mots clés Sulforaphane · Prostate · Cancer · PSA · Récidive · Prostatectomie totale
Effet du sulforaphane chez les hommes présentant une récidive biologique après prostatectomie totale, Bernard G. Cipolla, Eric Mandron, Jean Marc Lefort,Yves Coadou, Emmanuel Della Negra, Luc Corbel, Ronan Le Scodan, Abdel Rahmene Azzouzi and Nicolas Mottet, Oncologie (2016)
Prevention and Treatement of Chronic Disease / Summary of clinically relevant actions
SFN, which is the subject of this study, has been identified as a molecule with many interesting activities. In particular, the document covers the vast majority of the properties demonstrated for sulforaphanen and the related pathways, including in core cellular processes.
Abstract : A growing awareness of the mechanisms by which phytochemicals can influence upstream endogenous cellular defence processes has led to intensified research into their potential relevance in the prevention and treatment of disease. Pharmaceutical medicine has historically looked to plants as sources of the starting materials for drug development; however, the focus of nutraceutical medicine is to retain the plant bioactive in as close to its native state as possible. As a consequence, the potency of a nutraceutical concentrate or an extract may be lower than required for significant gene expression. The molecular structure of bioactive phytochemicals to a large extent determines the molecule’s bioavailability. Polyphenols are abundant in dietary phytochemicals, and extensive in vitro research has established many of the signalling mechanisms involved in favourably modulating human biochemical pathways. Such pathways are associated with core processes such as redox modulation and immune modulation for infection control and for downregulating the synthesis of inflammatory cytokines. Although the relationship between oxidative stress and chronic disease continues to be affirmed, direct-acting antioxidants such as vitamins A, C, and E, beta-carotene, and others have not yielded the expected preventive or therapeutic responses, even though several large meta-analyses have sought to evaluate the potential benefit of such supplements. Because polyphenols exhibit poor bioavailability, few of their impressive in vitro findings have been replicated in vivo. SFN, an aliphatic isothiocyanate, emerges as a phytochemical with comparatively high bioavailability. A number of clinical trials have demonstrated its ability to produce favourable outcomes in conditions for which there are few satisfactory pharmaceutical solutions, foreshadowing the potential for SFN as a clinically relevant nutraceutical. Although myrosinase-inert broccoli sprout extracts are widely available, there now exist myrosinase-active broccoli sprout supplements that yield sufficient SFN to match the doses used in clinical trials.
Summary of clinically relevant actions of SFN :
(1) Increases synthesis of glutathione. This has implications for oxidative stress and detoxification as glutathione is the substrate for both pathways. Glutathione is also an antioxidant in its own right.
(2) Inhibits some Phase 1 detoxification enzymes that activate chemical carcinogens. This reduces the level of toxic intermediates with carcinogenic potential. It also allows Phase 2 to “keep pace” with Phase 1 processing.
(3) Increases activity of Phase 2 detoxification enzymes. Sulforaphane is considered the most potent of the Phase 2 inducing substances. As a monofunctional inducer, sulforaphane is considered to be a significant component of the anticarcinogenic action of broccoli.
(4) Provides significant antioxidant activity, largely due to its ability to induce glutathione synthesis. Glutathione is a critical factor in protecting organisms against toxicity and disease. The ability of sulforaphane to upregulate glutathione synthesis is highly significant.
(5) Acts as a histone deacetylase inhibitor, providing DNA protection. Development of histone deacetylase inhibitors is a key avenue for cancer drug research.
(6) Induces apoptosis, inhibits MMP-2 (metastasis), and inhibits angiogenesis and cell cycle arrest (interacts at several levels). Therapeutic interventions which exhibit several related actions targeting the same underlying defect are considered highly desirable.
(7) Limits proinflammatory effects of diesel chemicals by upregulation of Phase 2 enzymes. Environmental pollutants are known to contribute to various
lung diseases. Removal of the toxins reduces tendency to disease.
(8) Induces thioredoxin (Trx) as part of the ARE. Thioredoxin is implicated in cardioprotection by triggering several survival proteins. Sulforaphane
may have beneficial effects in cardiovascular disease.
(9) Bactericidal against Helicobacter pylori and also blocks gastric tumour formation in animals. Helicobacter is known to contribute to development of stomach cancer. Elimination of the organism without the use of typical antimicrobial Triple Therapy could protect the colonic microflora.
(10) Protects dopaminergic cells from cytotoxicity and subsequent neuronal death (cell culture). Dopaminergic neurones are associated with Parkinson’s disease. Pharmaceuticals to treat Parkinsonism are not without risk and the disease is not usually detected until more than 50% of the neurones have been lost. A chemoprotective tool could prevent premature loss.
(11) Increases p-53 (associated with tumour suppression) and bax protein expression, thereby enhancing cellular protection against cancer. Sulforaphane is an attractive chemotherapeutic agent for tumours with a p53 mutation.
(12) Limits effect of aflatoxin on liver cells. Interventions which can offer significant protection against environmental and food-borne pollutants could prevent the consequences of these factors. Appropriate doses of sulforaphaneyielding substances are yet to be determined.
(13) Enhances natural killer cell activity and other markers of enhanced immune function. The immune system is a critical part of the body’s defences against inflammatory as well as infectious diseases. Most diseases benefit from enhancement to immune function.
(14) Suppresses NF-κB, a key regulator of inflammation. NF-κB expression is downregulated by sulforaphane and as such downregulates inducible proinflammatory enzymes such as cyclooxygenase (COX-2) and NO synthase (iNOS). As an inhibitor of NF-κB as well as an activator of Nrf2, SF modulates
many cancer-related events, including susceptibility to carcinogens, cell death, cell cycle, angiogenesis, invasion, and metastasis.
(15) Sulforaphane is not directly antioxidant. Instead, it exhibits a weak prooxidant effect. Because sulforaphane is not directly antioxidant but exerts its antioxidant effect primarily by induction of glutathione and other antioxidant compounds, it is considered to exhibit an indirect antioxidant effect.
(16) Potent inducer of HO-1 (haemoxygenase-1). Haemoxygenase-1 plays an important role in modulating the effects of oxidants in the lungs.
Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease, Christine A. Houghton, Hindawi - Oxidative Medicine and Cellular Longevity Volume 2019, Article ID 2716870, 27 pages
Anti-nociceptive and anti-inflammatory effects of sulforaphane on sciatic endometriosis
Evaluate the efficacy of sulforaphane in alleviating pain of sciatic nerve endometriosis. The effects of sulforaphane are mediated by activation of Keap1-Nrf2 signaling. This study supports further development of sulforaphane as a drug to treat sciatic nerve endometriosis without considerable side effects
Abstract : Endometriosis of sciatic nerve is a common gynecological disease. Here we aimed to study the anti-inflammatory and anti-nociceptive role of sulforaphane on sciatic nerve endometriosis. The sciatic nerve endometriosis rat model was constructed by autologous implantation of uterine tissue. Sulforaphane was administered intraperitoneally at the dose of 5, 15, 30 and 60 mg/kg/day for 28 days. Behavioral testing was performed at day 7, 14, 21 and 28. At day 28, rats were sacrificed, followed by collecting superficial dorsal horn tissues and lesions. Quantitative real-time PCR and Western blot were performed to assess COX2, Keap1, Nrf2 expression in collected tissues. Enzyme-linked immunosorbent assay was conducted to assess the expression of pro-inflammatory cytokines. Sulforaphane alleviated pain of sciatic endometriosis as evidenced by the increase in paw withdrawal threshold and paw withdrawal latency. Sulforaphane also inhibited ectopic endometrial tissue growth in sciatic endometriosis rat, shown as the shrinkage of lesion size and decreased VEGF levels. IL6, IL-1β and TNF-α levels were decreased by sulforaphane. Sulforaphane induced DOX2 and INOS suppression and Keap1 and Nrf2 upregulation. Sulforaphane alleviates pain induced by sciatic endometriosis, which is mediated by inhibiting inflammation.
Discussions : While inflammation and chronic pain are two major symptoms of sciatic nerve endometriosis, current treatments have yet to efficiently attenuate these symptoms. In addition, current drugs, such as non-steroidal anti-inflammatory drugs, opioid drugs and hormone drugs, are of considerable side effects, limiting their application in the disease [6]. The use of dietary compounds, such as sulforaphane reported in the present study, for pain and inflammation attenuation is highly desirable. Emerging
Conclusions : In summary, here we have constructed a sciatic nerve endometriosis model in rats and evaluated the therapeutic effects of sulforaphane. We have showed that sulforaphane successfully alleviated pain and inflammation, reducing lesion size. The effects of sulforaphane are mediated by activation of Keap1-Nrf2 signaling. Our study supports further development of sulforaphane as a drug to treat sciatic nerve endometriosis without considerable side effects.
Keywords: Inflammation; Keap1; Nrf2; Sciatic nerve endometriosis; Sulforaphane
Anti-nociceptive and anti-inflammatory effects of sulforaphane on sciatic endometriosis in a rat model - Yan Liu, Zhiwei Zhang, Xiaofen Lu, Jian Meng, Xuying Qin, Jie Jiang - Neuroscience Letters Volume 723, 1 April 2020, 134858
Sulforaphane Attenuates Endometriosis in Rat Models
The present study, for the first time, identified that sulforaphane ameliorated endometriosis in rat models by multiple effects.
Abstract : Sulforaphane exerts anti-inflammatory activity in inflammatory diseases. The endometriosis (EM) is accompanied by chronic inflammation. The present study aims to explore the therapeutic effects of sulforaphane on EM and its underlying mechanism. An EM rat model was established by transplantation of autologous fragments. The rats were intragastrically administered sulforaphane (5 mg/kg, 15 mg/kg, and 30 mg/kg) for 3 weeks. The volumes of endometriotic foci and adhesion score were calculated at the end of the experiment. Levels of interleukin (IL)-6, IL-10, tumor necrosis factor (TNF)-a, interferon (IFN)-g, and vascular endothelial growth factor (VEGF) were determined by enzyme-linked immunosorbent assay (ELISA). Expressions of VEGF, B-cell lymphoma/leukemia 2 (Bcl-2), Bax, cleaved caspase-3, PI3K, and Akt in endometrial tissue were determined by Western blotting. Relative expressions of PI3K and Akt were determined by quantitative polymerase chain reaction. Posttreatment of sulforaphane dose-dependently decreased the volumes of endometriotic foci and adhesion score in EM model. Additionally, posttreatment of sulforaphane inhibited levels of IL-6, IL-10, TNF-a, IFN-g, and VEGF in peritoneal fluid and plasma. Posttreatment of sulforaphane regulated the expressions of VEGF, bcl-2, Bax, and cleaved Caspase-3 in EM model. The underlying mechanism revealed that sulforaphane attenuated EM in the rat model by inhibition of PI3K/Akt signaling pathway.
Keywords : sulforaphane, inflammation, apoptosis, endometriosis, VEGF, PI3K/Akt
Discussion : Endometriosis (EM) is a chronic disease characterized by endometrial-like tissue outside the uterine cavity. It has been recognized to be associated with pelvic pain and impaired fecundity in adolescents.2 There are 6% to 10% of women with reproductive age, 50% to 60% of women with pelvic pain, and as many as 50% of women with infertility affected by EM. Inflammation has been reported to play an important role in the development of EM.
Conclusion : The present study, for the first time, identified that sulforaphane ameliorated EM in rat models by multiple effects. First, treatment of sulforaphane decreased levels of inflammatory cytokines including TNF-a and IL-6 in peritoneal fluid and plasma. Second, treatment of sulforaphane decreased the expressions of VEGF in the peritoneal fluid, plasma, and ectopic endometria. Third, treatment of sulforaphane regulated the expressions of apoptosis-related biomarkers including Bcl2, Bax, and cleaved Caspase-3. Moreover, we revealed that sulforaphane-ameliorated EM in rat models is by inhibition of PI3K/Akt signaling pathway.
Sulforaphane Attenuates Endometriosis in Rat Models Through Inhibiting PI3K/Akt Signaling Pathway, Aixiu Zhou, Yiting Hong, and Yuchun Lv, DOI: 10.1177/1559325819855538, Dose-Response: An International Journal April-June 2019:1-8
Sulforaphane in cancer chemoprevention and health benefits
In this paper, we briefly review structure, pharmacology and preclinical studies highlighting chemopreventive effects of SFN.
Abstract Cancer is a multi-stage process resulting from aberrant signaling pathways driving uncontrolled proliferation of transformed cells. The development and progression of cancer from a premalignant lesion towards a metastatic tumor requires accumulation of mutations in many regulatory genes of the cell. Different chemopreventative approaches have been sought to interfere with initiation and control malignant progression. Here we present research on dietary compounds with evidence of cancer prevention activity that highlights the potential beneficial effect of a diet rich in cruciferous vegetables. The Brassica family of cruciferous vegetables such as broccoli is a rich source of glucosinolates, which are metabolized to isothiocyanate compounds. Amongst a number of related variants of isothiocyanates, sulforaphane (SFN) has surfaced as a particularly potent chemopreventive agent based on its ability to target multiple mechanisms within the cell to control carcinogenesis. Anti-inflammatory, pro-apoptotic and modulation of histones are some of the more important and known mechanisms by which SFN exerts chemoprevention. The effect of SFN on cancer stem cells is another area of interest that has been explored in recent years and may contribute to its chemopreventive properties. In this paper, we briefly review structure, pharmacology and preclinical studies highlighting chemopreventive effects of SFN.
Keywords Chemopreventive agents . Isothiocyanates . Sulforaphane
The role of Sulforaphane in cancer chemoprevention and health benefits: a mini-review Reza Bayat Mokhtari, Narges Baluch, Tina S. Homayouni, Evgeniya Morgatskaya, Sushil Kumar, Parandis Kazemi, Herman Yeger, Published online: 23 July 2017, J. Cell Commun. Signal. (2018) 12:91–101
Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue
Sulforaphane (SFN) is an emerging nutraceutical, a functional ingredient that provides health benefits, and is potentially helpful for preventing and/or treating diseased conditions. There is also evidence on the use of SFN as a cancer chemopreventive compound, due to its potential anticarcinogenic and antioxidative properties. SFN can also produce synergistic effects and prevent inflammation and protein expression of inflammatory enzymes, such as iNOS and COX-2, in combination with other functional ingredients, such as nobiletin (NBN). In addition, compared with other functional bioactive compounds, SFN can rapidly induce phase 2 enzyme activity in bladder tissues
Abstract: Cruciferous vegetables hold a myriad of bioactive molecules that are renowned for possessing unique medicinal benefits. Sulforaphane (SFN) is one of the potential nutraceuticals contained within cruciferous vegetables that is useful for improving health and diseased conditions. The objective of this review is to discuss the mechanistic role for SFN in preventing oxidative stress, fatigue, and inflammation. Direct and indirect research evidence is reported to identify the nontoxic dose of SFN for human trials, and effectiveness of SFN to attenuate inflammation and/or oxidative stress. SFN treatment modulates redox balance via activating redox regulator nuclear factor E2 factor-related factor (Nrf2). SFN may play a crucial role in altering the Keap1/Nrf2/ARE pathway (an intricate response to many stimuli or stress), which induces Nrf2 target gene activation to reduce oxidative stress. In addition, SFN reduces inflammation by suppressing centrally involved inflammatory regulator nuclear factor-kappa B (NF-KB), which in turn downregulates the expression of proinflammatory cytokines and mediators. Exercise may induce a significant range of fatigue, inflammation, oxidative stress, and/or organ damage due to producing excessive reactive oxygen species (ROS) and inflammatory cytokines. SFN may play an effective role in preventing such damage via inducing phase 2 enzymes, activating the Nrf2/ARE signaling pathway or suppressing nuclear translocation of NF-KB. In this review, we summarize the integrative role of SFN in preventing fatigue, inflammation, and oxidative stress, and briefly introduce the history of cruciferous vegetables and the bioavailability and pharmacokinetics of SFN reported in previous research. To date, very limited research has been conducted on SFN’s effectiveness in improving exercise endurance or performance. Therefore, more research needs to be carried out to determine the effectiveness of SFN in the field of exercise and lifestyle factors.
Keywords: cruciferous vegetables; sulforaphane; reactive oxygen species; Nrf2; inflammation; NF- KB; exercise; organ damage, brocoli, broccoli
The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical Ruheea Taskin Ruhee and Katsuhiko Suzuki, Published: 13 June 2020, Antioxidants 2020, 9, 521
Sulforaphane for the treatment of chronic inflammatory diseases
Cruciferous vegetables contain the precursor glucoraphanin, which is hydrolysed upon consumption to form L-sulforaphane (LSF), the primary active compound that mediates potential cardio-protective and anti-carcinogenic effects. This review discusses the clinical evidence to date in relation to the use of LSF in the context of chronic inflammatory diseases as well as provide key mechanistic insights for these effects
Summary : According to the World Health Organisation, 70% of all deaths globally can be attributed to chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, respiratory conditions, cardiovascular diseases, diabetes and cancer. Chronic inflammation has a significant impact on the quality of life of affected individuals with an increased risk of developing other chronic inflammatory diseases. Given the limitations of current pharmaceuticals, there is an intense research interest in identifying novel dietary interventions that can regulate and alleviate inflammation. A diet rich in cruciferous vegetables has been extensively studied for its immediate and long-term health benefits, particularly in the context of cardiovascular disease and cancer. Cruciferous vegetables contain the precursor glucoraphanin, which is hydrolysed upon consumption to form L-sulforaphane (LSF), the primary active compound that mediates potential cardio-protective and anti-carcinogenic effects. LSF has been shown to have beneficial effects in vitro and in animal studies through its classical antioxidant and anti-inflammatory properties, and more recently its chromatin modifying effects. This review discusses the clinical evidence to date in relation to the use of LSF in the context of chronic inflammatory diseases as well as provide key mechanistic insights for these effects.
Conclusion : To date, clinical studies with LSF or relevant precursorcontaining extracts have yielded promising yet, inconsistent results. These are largely due to differences in the study designs, source (bioactivity), and dose of LSF, and the disease context and/ or target population studied. Nevertheless, the beneficial effects observed in certain diseases and the lack of serious side effects observed to date is exciting. Therefore, the potential for LSF to mediate important clinical benefits in those with chronic inflammatory diseases is promising although further clinical studies are warranted.
The potential use of L-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence Nadia Mazarakis, Kenneth Snibson, Paul V. Licciardi, Tom C. Karagiannis, Clinical Nutrition 39 (2020) 664-675
Epigenetic regulation of bone remodeling
Sulforaphane increases bone formation and reduces bone resorption by promoting osteoblast differentiation
Abstract : Osteoporosis and osteopenia impact more than 54 million Americans, resulting in significant morbidity and mortality. Alterations in bone remodeling are the hallmarks for osteoporosis, and thus the development of novel treatments that will prevent or treat bone diseases would be clinically significant, and improve the quality of life for these patients. Bone remodeling involves the removal of old bone by osteoclasts and the formation of new bone by osteoblasts. This process is tightly coupled, and is essential for the maintenance of bone strength and integrity. Since the osteoclast is the only cell capable of bone resorption, the development of drugs to treat bone disorders has primarily focused on reducing osteoclast differentiation, maturation, and bone resorption mechanisms, and there are few treatments that actually increase bone formation. Evidence from observational, experimental, and clinical studies demonstrate a positive link between naturally occurring compounds and improved indices of bone health. While many natural extracts and compounds are reported to have beneficial effects on bone, only resveratrol, sulforaphane, specific phenolic acids and anthocyanins, have been shown to both increase bone formation and reduce resorption through their effects on the bone epigenome. Each of these compounds alters specific aspects of the bone epigenome to improve osteoblast differentiation, reduce osteoblast apoptosis, improve bone mineralization, and reduce osteoclast differentiation and function. This review focuses on these specific natural compounds and their epigenetic regulation of bone remodeling.
Keywords : anthocyanins; DNA methylation; ferulic acid; non-coding RNA; osteoblast; osteoclast; resveratrol; sulforaphane; syringic acid
Discussion : In bone, SFN mimics dimethyl sulfoxide (DMSO), as DMSO is known to cause phenotypic changes and differentiation of osteoblasts by acting on the epigenome. Sulforaphane increases bone formation and reduces bone resorption by promoting osteoblast differentiation via epigenetic alterations in DNA methylation and alterations of gene expression. Sulforaphane also induced pre-osteoclast apoptosis.
Epigenetic regulation of bone remodeling by natural compounds Nishikant Raut, Sheila M. Wicks, Tempitope O. Lawal, Gail B. Mahady, Pharmacol Res. 2019 September ; 147: 104350. doi:10.1016/j.phrs.2019.104350
Antioxidant and cytoprotective effects of SFN in brain endothelial cells
Brain oxidative stress during
inflammation and excitotoxicity leads to neurovascular injury. We tested the hypothesis
that SNF exhibits acute antioxidant effects and prevents neurovascular injury
during oxidative stress.
Abstract Sulforaphane (SFN), a bioactive phytochemical isothiocyanate, has a wide spectrum of cytoprotective effects that involve induction of antioxidant genes. Nongenomic antioxidant effects of SFN have not been investigated. Brain oxidative stress during inflammation and excitotoxicity leads to neurovascular injury. We tested the hypothesis that SNF exhibits acute antioxidant effects and prevents neurovascular injury during oxidative stress. In primary cultures of cerebral microvascular endothelial cells (CMVEC) and cortical astrocytes from the newborn pig brain, a pro-inflammatory cytokine TNF-α and an excitotoxic glutamate elevate reactive oxygen species (ROS) and cause cell death by apoptosis. Nox4 NADPH oxidase is the main Nox isoform in CMVEC and cortical astrocytes that is acutely activated by TNF-α and glutamate leading to ROS-mediated cell death by apoptosis. The Nox4 inhibitor GKT137831 blocked NADPH oxidase activity and overall ROS elevation, and prevented apoptosis of CMVEC and astrocytes exposed to TNF-α and glutamate, supporting the leading role of Nox4 in the neurovascular injury. Synthetic SFN (10−11-10−6 mol/L) inhibited NADPH oxidase activity and reduced overall ROS production in CMVEC and astrocytes within 1-hour exposure to TNF-α and glutamate. Furthermore, in the presence of SFN, the ability of TNF-α and glutamate to produce apoptosis in CMVEC and cortical astrocytes was completely prevented. Overall, SFN at low concentrations exhibits antioxidant and antiapoptotic effects in cerebral endothelial cells and cortical astrocytes via a via a nongenomic mechanism that involves inhibition of Nox4 NADPH oxidase activity. SFN may prevent cerebrovascular injury during brain oxidative stress caused by inflammation and glutamate excitotoxicity.
KEYWORDS antioxidants, apoptosis, astrocytes, endothelial cells, excitotoxicity, inflammation, isothiocyanates, neurovascular unit, newborn pigs, oxidative stress, primary cells, sulforaphane
Acute antioxidant and cytoprotective effects of sulforaphane in brain endothelial cells and astrocytes during inflammation and excitotoxicity, Jianxiong Liu, Pharmacol Res Perspect. 2020;e00630
Sulforaphane as a Natural Immune System Enhancer
Brassicaceae are an outstanding source of bioactive compounds such as ascorbic acid, polyphenols, essential minerals, isothiocyanates and their precursors, glucosinolates (GSL). Recently, GSL gained great attention because of the health promoting properties of their hydrolysis products: isothiocyanates. Among them, sulforaphane (SFN) became the most attractive one.
Abstract: Brassicaceae are an outstanding source of bioactive compounds such as ascorbic acid, polyphenols, essential minerals, isothiocyanates and their precursors, glucosinolates (GSL). Recently, GSL gained great attention because of the health promoting properties of their hydrolysis products: isothiocyanates. Among them, sulforaphane (SFN) became the most attractive one owing to its remarkable health-promoting properties. SFN may prevent different types of cancer and has the ability to improve hypertensive states, to prevent type 2 diabetes–induced cardiomyopathy, and to protect against gastric ulcer. SFN may also help in schizophrenia treatment, and recently it was proposed that SFN has potential to help those who struggle with obesity. The mechanism underlying the health-promoting effect of SFN relates to its indirect action at cellular level by inducing antioxidant and Phase II detoxifying enzymes through the activation of transcription nuclear factor (erythroidderived 2)-like (Nrf2). The effect of SFN on immune response is generating scientific interest, because of its bioavailability, which is much higher than other phytochemicals, and its capacity to induce Nrf2 target genes. Clinical trials suggest that sulforaphane produces favorable results in cases where pharmaceutical products fail. This article provides a revision about the relationship between sulforaphane and immune response in different diseases. Special attention is given to clinical trials related with immune system disorders.
Keywords: sulforaphane; immunological response; cellular mechanism
Potential of Sulforaphane as a Natural Immune System Enhancer: A Review, Andrea Mahn, and Antonio Castillo, Molecules 2021, 26, 752
Antiviral activity against CoV-2 and seasonal HCoV-OC43 coronaviruses
SFN can inhibit in vitro and in vivo replication of SARS-CoV-2 at pharmacologically and potentially therapeutically achievable concentrations. Further, it can modulate the inflammatory response, thereby decreasing the consequences of infection in mice when administered prior to infection. Given that SFN is orally bioavailable, commercially available, and has limited side effects, our results suggest it could be a promising approach for the prevention and treatment of COVID-19 as well as other coronavirus infections. Further studies are needed to address these possibilities.
Abstract : Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has incited a global health crisis. Currently, there are limited therapeutic options for the prevention and treatment of SARS-CoV-2 infections. We evaluated the antiviral activity of sulforaphane (SFN), the principal biologically active phytochemical derived from glucoraphanin, the naturally occurring precursor present in high concentrations in cruciferous vegetables. SFN inhibited in vitro replication of six strains of SARS-CoV-2, including Delta and Omicron, as well as that of the seasonal coronavirus HCoVOC43. Further, SFN and remdesivir interacted synergistically to inhibit coronavirus infection in vitro. Prophylactic administration of SFN to K18-hACE2 mice prior to intranasal SARS-CoV- 2 infection significantly decreased the viral load in the lungs and upper respiratory tract and reduced lung injury and pulmonary pathology compared to untreated infected mice. SFN treatment diminished immune cell activation in the lungs, including significantly lower recruitment of myeloid cells and a reduction in T cell activation and cytokine production. Our results suggest that SFN should be explored as a potential agent for the prevention or treatment of coronavirus infections.
Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice Alvaro A. Ordonez, COMMUNICATIONS BIOLOGY
Sulforaphane increases Nrf2 expression and protects alveolar epithelial cells against injury caused by cigarette smoke extract
Cigarette smoking is a primary risk factor for chronic obstructive pulmonary disease (COPD). The present study was undertaken to investigate the effects and underlying mechanisms of SFN in preventing cigarette smoke extract (CSE)‑induced oxidative damage to RLE‑6TN rat lung epithelial cells.
Abstract. Cigarette smoking is a primary risk factor for chronic obstructive pulmonary disease (COPD), as it damages epithelial cells through a variety of mechanisms. Sulforaphane (SFN) is an antioxidant agent, which exerts protective effects against cell damage by activating the nuclear factor erythroid 2 like 2 (NFE2L2; Nrf2). The present study was undertaken to investigate the effects and underlying mechanisms of SFN in preventing cigarette smoke extract (CSE)‑induced oxidative damage to RLE‑6TN rat lung epithelial cells. MTT assay was used to determine the cytotoxicity of SFN and CSE. The effect of SFN and CSE on cell cycle progression, apoptosis and intracellular reactive oxygen species (ROS) levels were analyzed using flow cytometry. Reverse transcription‑quantitative polymerase chain reaction and western blotting were used to quantify mRNA and protein expression levels of Nrf2 respectively. SFN protected RLE‑6TN cells from oxidative damage, potentially via increasing Nrf2 expression and reducing ROS levels. In addition, SFN attenuated G1 phase cell cycle arrest and abrogated apoptosis. Therefore, SFN protected alveolar epithelial cells against CSE‑induced oxidative injury by upregulating Nrf2 expression. The results of the present study may provide theoretical support for the clinical use of SFN in patients with COPD.
In conclusion, the results of the present study indicate that CSE inhibits RLE‑6TN cell proliferation and induces apoptosis. Pretreatment with SFN may alleviate cell injury and reduce ROS damage, potentially via increased Nrf2 expression. These results suggest that Nrf2 may be a potential therapeutic target for the treatment of COPD.
Keywords: cigarette smoke extract, alveolar epithelial cells, sulforaphane, nuclear factor erythroid 2 like 2
Sulforaphane increases Nrf2 expression and protects alveolar epithelial cells against injury caused by cigarette
smoke extract, ZONGXIAN JIAO, MOLECULAR MEDICINE REPORTS 16: 1241-1247, 2017
Dose-dependent detoxication of the airborne pollutant benzene
An intervention with a broccoli sprout beverage enhanced the detoxication of benzene, an important airborne pollutant.
Background: Airborne pollutants have collectively been classified as a known human carcinogen and, more broadly, affect the health of hundreds of millions of people worldwide. Benzene is a frequent component of air pollution, and strategies to protect individuals against unavoidable exposure to this and other airborne carcinogens could improve the public’s health. Earlier clinical trials in Qidong, China, demonstrated efficacy in enhancing the detoxication of benzene using a broccoli sprout beverage.
Objectives: A randomized, placebo-controlled, multidose trial of a broccoli sprout beverage was designed to determine the lowest effective concentration that enhances benzene detoxication adjudged by enhanced excretion of the urinary biomarker, S-phenylmercapturic acid (SPMA).
Methods: Following informed consent, 170 subjects were randomly assigned in 5 blocks of 34 each to drink either a placebo beverage (n=55) or 1 of 3 graded concentrations of a broccoli sprout beverage [full (n = 25), one-half (n = 35), and one-fifth (n = 55)] for 10 consecutive days. Concentrations of SPMA arising through induced benzene conjugation with glutathione were quantified by MS in sequential 12-h overnight urine collections during the intervention.
Results: MS was also used to quantify urinary sulforaphane metabolites in each dosing regimen that resulted in a median 24-h urinary output of 24.6, 10.3, and 4.3 μmol, respectively, confirming a dose-dependent de-escalation of the inducing principle within the beverage. A statistically significant increase in benzene mercapturic acids in urinewas found for the high-dose group (+63.2%) during the 10-d period. The one-half dose (+11.3%) and one-fifth dose groups (−6.4%) were not significantly different from placebo controls.
Conclusions: An intervention with a broccoli sprout beverage enhanced the detoxication of benzene, an important airborne pollutant, when dosed at a concentration evoking a urinary elimination of ∼25 μmol sulforaphane metabolites per day, and it portends a practical and frugal population-based strategy to attenuate associated long-term health risks of air pollution. This trial was registered at clinicaltrials.gov as NCT02656420
Keywords: broccoli, sulforaphane, glucoraphanin, air pollution, mercapturic acids, randomized clinical trial
Dose-dependent detoxication of the airborne pollutant benzene in a randomized trial of broccoli sprout beverage in Qidong, China, Jian-Guo Che, Am J Clin Nutr 2019;110:675–684
Sulforaphane Activates a lysosome-dependent transcriptional program to mitigate oxidative stress
By simultaneously activating macroautophagy/autophagy and detoxifying pathways, natural compound SFN may trigger a self-defense cellular mechanism that can effectively mitigate oxidative stress commonly associated with many metabolic and age-related diseases
ABSTRACT : Oxidative stress underlies a number of pathological conditions, including cancer, neurodegeneration, and aging. Antioxidant-rich foods help maintain cellular redox homeostasis and mitigate oxidative stress, but the underlying mechanisms are not clear. For example, sulforaphane (SFN), an electrophilic compound that is enriched in cruciferous vegetables such as broccoli, is a potent inducer of cellular antioxidant responses. NFE2L2/NRF2 (nuclear factor, erythroid 2 like 2), a transcriptional factor that controls the expression of multiple detoxifying enzymes through antioxidant response elements (AREs), is a proposed target of SFN. NFE2L2/NRF2 is a target gene of TFEB (transcription factor EB), a master regulator of autophagic and lysosomal functions, which we show here to be potently activated by SFN. SFN induces TFEB nuclear translocation via a Ca2+- dependent but MTOR (mechanistic target of rapamycin kinase)-independent mechanism through a moderate increase in reactive oxygen species (ROS). Activated TFEB then boosts the expression of genes required for autophagosome and lysosome biogenesis, which are known to facilitate the clearance of damaged mitochondria. Notably, TFEB activity is required for SFN-induced protection against both acute oxidant bursts and chronic oxidative stress. Hence, by simultaneously activating macroautophagy/autophagy and detoxifying pathways, natural compound SFN may trigger a self-defense cellular mechanism that can effectively mitigate oxidative stress commonly associated with many metabolic and age-related diseases.
KEYWORDS ; Autophagy; lysosome; NFE2L2/NRF2; ROS; sulforaphane; TFEB
Sulforaphane Activates a lysosome-dependent transcriptional program to mitigate oxidative stress, - Dan Li, Rong Shao, Na Wang, Nan Zhou, Kaili Du... - AUTOPHAGY 2021, VOL. 17, NO. 4, 872–887
Protective Effect of Sulforaphane against Oxidative Stress in Human Granulosa Cells
SFN protects human GCs against H2O2 induced-Oxydative Stress, a potential application in assisted reproduction cycles by improving the quality of GCs and the embedded oocyte, especially in PCOS patients.
Introduction : It has been revealed that ROS plays a regulatory role in the female reproductive system, especially during folliculogenesis, steroidogenesis, oocyte maturation, and luteolysis.
Objective: Sulforaphane (SFN) is a natural free radical scavenger that can reduce oxidative stress (OS) through mediating nuclear factor (erythroid-derived 2)-like 2 (NF-E2-related factor 2 or NRF2)/antioxidant response element (ARE) signaling pathway and the downstream antioxidant enzymes. Here, we intended to study the role of SFN in OSinduced human granulosa cells (GCs) by investigating the intracellular levels of reactive oxygen species (ROS), cell death, and NRF2-ARE pathway.
Materials and Methods: This experimental study was conducted on GCs of 12 healthy women who had normal menstrual cycles with no history of polycystic ovary syndrome (PCOS), endometriosis, menstrual disorders, hyperprolactinemia, or hormonal therapy. After isolation of GCs, the MTT assay was performed to explore GCs viability after treatment with SFN in the presence or absence of H2O2. Flow cytometry was utilized to determine the intracellular ROS production and the apoptosis rate. Evaluation of the mRNA and protein expression levels of NRF2 and phase II enzymes including superoxide dismutase (SOD) and catalase (CAT) was performed by quantitative real-time polymerase chain reaction (PCR) and western blotting. Finally, the data were analyzed by SPSS software using One-way ANOVA and the suitable post-hoc test. Significance level was considered as P max 0.05.
Results: Pretreatment of GCs with SFN attenuated intracellular ROS production and apoptosis rate in the H2O2-exposed cells. Moreover, SFN treatment increased the mRNA expression level of NRF2, SOD, and CAT. Higher expression of NRF2 and SOD was also observed at the protein level.
Conclusion: Our study demonstrated that SFN protects human GCs against H2O2 induced-OS by reducing the intracellular ROS production and the following apoptosis through a mechanism by which NRF2 increases the antioxidant enzymes such as SOD and CAT. This result may have a potential application in assisted reproduction cycles by improving the quality of GCs and the embedded oocyte, especially in PCOS patients.
The present study indicated that SFN induces the expression of NRF2 and its downstream antioxidant enzymes, SOD and CAT, at both gene and protein expression levels in human GCs under OS conditions. Moreover, SFN reduces the levels of intracellular ROS and the apoptosis rate of the GCs. It is tempting to speculate that the stimulation of the NRF2-ARE pathway by SFN attenuates the damage by OS in human GCs via the activation of SOD and CAT. Hence, this study may have applicable information for improving the outcomes of assisted reproduction cycles, especially in PCOS patients
Keywords: Granulosa Cells, NF-E2-Related Factor 2, Oxidative Stress, Sulforaphane
The Protective Effect of Sulforaphane against Oxidative Stress through Activation of NRF2/ARE Pathway in Human Granulosa Cells, Sahar Esfandyari, Cell Journal(Yakhteh), Vol 23, No 6, November 2021, Pages: 692-700
New highlights on the health-improving effects of sulforaphane (different diseases, mainly diabetes and cancer)
Recent evidence about the beneficial effects of sulforaphane (SFN), which is the most studied member of isothiocyanates, on both in vivo and in vitro models of different diseases, mainly diabetes and cancer.
Abstract : In this paper, we review recent evidence about the beneficial effects of sulforaphane (SFN), which is the most studied member of isothiocyanates, on both in vivo and in vitro models of different diseases, mainly diabetes and cancer. The role of SFN on oxidative stress, inflammation, and metabolism is discussed, with emphasis on those nuclear factor E2-related factor 2 (Nrf2) pathway-mediated mechanisms. In the case of the anti-inflammatory effects of SFN, the point of convergence seems to be the downregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), with the consequent amelioration of other pathogenic processes such as hypertrophy and fibrosis. We emphasized that SFN shows opposite effects in normal and cancer cells at many levels; for instance, while in normal cells it has protective actions, in cancer cells it blocks the induction of factors related to the malignity of tumors, diminishes their development, and induces cell death. SFN is able to promote apoptosis in cancer cells by many mechanisms, the production of reactive oxygen species being one of the most relevant ones. Given its properties, SFN could be considered as a phytochemical at the forefront of natural medicine.
Conclusion : Accumulated evidence has shown the beneficial effects of SFN on health, although its pharmacological mechanisms are very diverse and they have not been completely elucidated. It has been shown that its protective effect is directly related to the induction of the Nrf2 pathway, which activates several genes involving the antioxidant system; it also possesses anti-inflammatory effects, enhances the metabolism of many substrates and modulates mitochondrial function. SFN can protect the organism against excessive ROS production by inducing some antioxidant enzymes such as HO-1, NQO1, SOD, catalase, and glutathione peroxidase. Furthermore, SFN can also protect against cell death by blocking some pro-apoptotic proteins, decreasing ER stress and modulating autophagy. Inflammation is also a key feature in the progression of plenty of chronic diseases; SFN can exert a protective effect by hindering the NF-κB pathway, and thus, it can also prevent the induction of pro-inflammatory cytokines and prevent the remote responses to it.
New highlights on the health-improving effects of sulforaphane - Alfredo Briones-Herrera, Dianelena Eugenio-Pérez, Jazmin Gabriela Reyes-Ocampo, Susana Rivera-Mancía and José Pedraza-Chaverri; DOI: 10.1039/c8fo00018b, The Royal Society of Chemistry 2018, Food Funct., 2018, 9, 2589–2606 / 2589
Chemopreventive activity of sulforaphane
Cancer is one of the major causes of morbidity and mortality in the world. Some studies suggest that cruciferous vegetable intake may lower overall cancer risk, including colon, lung, and prostate cancer, particularly during the early stages.Sulforaphane (SFN), which is converted from a major glucosinolate in broccoli/broccoli sprouts, has been shown to prevent chemically induced cancers in animal models and to inhibit the growth of established tumors.
Abstract: Cancer is one of the major causes of morbidity and mortality in the world. Carcinogenesis is a multistep process induced by genetic and epigenetic changes that disrupt pathways controlling cell proliferation, apoptosis, differentiation, and senescence. In this context, many bioactive dietary compounds from vegetables and fruits have been demonstrated to be effective in cancer prevention and intervention. Over the years, sulforaphane (SFN), found in cruciferous vegetables, has been shown to have chemopreventive activity in vitro and in vivo. SFN protects cells from environmental carcinogens and also induces growth arrest and/or apoptosis in various cancer cells. In this review, we will discuss several potential mechanisms of the chemopreventive activity of SFN, including regulation of Phase I and Phase II drug-metabolizing enzymes, cell cycle arrest, and induction of apoptosis, especially via regulation of signaling pathways such as Nrf2-Keap1 and NF-KB. Recent studies suggest that SFN can also affect the epigenetic control of key genes and greatly influence the initiation and progression of cancer. This research may provide a basis for the clinical use of SFN for cancer chemoprevention and enable us to design preventive strategies for cancer management, reduce cancer development and recurrence, and thus improve patient survival.
Keywords: sulforaphane, tumor, chemoprevention, Phase I and Phase II drug-metabolizing enzymes, apoptosis, anti-inflammatory, cell cycle progression, epigenetics
Chemopreventive activity of sulforaphane - Xin Jiang - Drug Design, Development and Therapy, 2018:12 2905–2913
Sulforaphane and Other Nutrigenomic Nrf2 Activators
The evolving science of nutrigenomics is in many ways legitimizing the important role of plant foods in human health. Of the many thousands of phytochemicals in the food supply, sulforaphane exhibits properties which maymake it an ideal cytoprotective biomolecule, deliverable in practical doses as a whole food supplement.
Abstract : The recognition that food-derived nonnutrient molecules can modulate gene expression to influence intracellular molecular mechanisms has seen the emergence of the fields of nutrigenomics and nutrigenetics. The aim of this review is to describe the properties of nutrigenomic activators of transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2), comparing the potential for sulforaphane and other phytochemicals to demonstrate clinical efficacy as complementarymedicines. Broccoli-derived sulforaphane emerges as a phytochemical with this capability, with oral doses capable of favourably modifying genes associated with chemoprevention. Compared with widely used phytochemical-based supplements like curcumin, silymarin, and resveratrol, sulforaphane more potently activates Nrf2 to induce the expression of a battery of cytoprotective genes. By virtue of its lipophilic nature and low molecular weight, sulforaphane displays significantly higher bioavailability than the polyphenol-based dietary supplements that also activate Nrf2. Nrf2 activation induces cytoprotective genes such as those playing key roles in cellular defense mechanisms including redox status and detoxification. Both its high bioavailability and significantNrf2 inducer capacity contribute to the therapeutic potential of sulforaphane-yielding supplements.
Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? - Christine A. Houghton - Hindawi Publishing Corporation Oxidative Medicine and Cellular Longevity Volume 2016, Article ID 7857186, 17 pages
Korean scientists have indicated that sulforaphane could help prevent hair loss and even boost hair growth.
Sulforaphane could not only become an unmissable ingredient in hair care cosmetics, but even an alternative pharmacological treatment for androgenetic alopecia, of which baldness is the ultimate stage. This is the finding of a team of scientists from South Korean company Gragem Co. Ltd. and of the Department of Biotechnology at The University of Suwon.
Abstract: Sulforaphane increases the expression of the dihydrotestosterone (DHT)-degrading enzyme, 3 α hydroxysteroid dehydrogenase (3 α HSD) in the liver, which accelerates DHT degradation, thereby inhibiting hair loss in the animal model. In this study, we elucidated its underlying mechanism and demonstrated that sulforaphane has hair loss inhibitory functions in RAW264.7 macrophage cells and Hepa1c1c7 cells at the cellular and gene levels. The gene expression level of an isoform of 3 α HSD, Akr1c2, increased in a dose-dependent manner when these cells were treated with sulforaphane, but there were no significant differences at the gene levels of Akr1c2 and Dhrs9 for the negative control mixture of biotin, dexpanthenol, and L-menthol. These studies indicated that sulforaphane is involved in regulating the gene expression of Akr1c2. To further determine whether this hair product has effects on alleviating hair loss symptoms, clinical trials were also conducted for 18 weeks. We performed a visual evaluation of the parietal and frontal lines of 23 patients before and after using the product, and then calculated the total number of hairs. This clinical study showed that the parietal lines and bangs visually improved and the number of hairs increased by 6.71% from before using the test product to 18 weeks after using the test product. Taken together, these cellular and clinical studies strongly suggest that sulforaphane may be an active ingredient that significantly alleviates hair loss symptoms.
Keywords: sulforaphane; hair loss; cosmetic ingredient; functional cosmetics
Sulforaphane, L-Menthol, and Dexpanthenol as a Novel Active Cosmetic Ingredient Composition for RelievingHair Loss Symptoms Youngkum Park, Published: 30 June 2021, Cosmetics 2021, 8, 63
Anticancer Activity of Sulforaphane
Human clinical studies have supported the chemopreventive effects of SFN on carcinogenesis. Based on the above-mentioned studies, it is clear that the dietary compound SFN, which has little or no adverse side effects, exerts anticancer activities through multiple mechanisms, including epigenetic regulation. Thus, daily consumption of cruciferous vegetables rich in SFN is not only a healthy diet choice but also an effective chemopreventive strategy.
Abstract : Sulforaphane (SFN), a compound derived from cruciferous vegetables that has been shown to be safe and nontoxic, with minimal/ no side effects, has been extensively studied due to its numerous bioactivities, such as anticancer and antioxidant activities. SFN exerts its anticancer effects by modulating key signaling pathways and genes involved in the induction of apoptosis, cell cycle arrest, and inhibition of angiogenesis. SFN also upregulates a series of cytoprotective genes by activating nuclear factor erythroid-2- (NF-E2-) related factor 2 (Nrf2), a critical transcription factor activated in response to oxidative stress; Nrf2 activation is also involved in the cancer-preventive effects of SFN. Accumulating evidence supports that epigenetic modification is an important factor in carcinogenesis and cancer progression, as epigenetic alterations often contribute to the inhibition of tumor-suppressor genes and the activation of oncogenes, which enables cells to acquire cancer-promoting properties. Studies on the mechanisms underlying the anticancer effects of SFN have shown that SFN can reverse such epigenetic alterations in cancers by targeting DNA methyltransferases (DNMTs), histone deacetyltransferases (HDACs), and noncoding RNAs. Therefore, in this review, we will discuss the anticancer activities of SFN and its mechanisms, with a particular emphasis on epigenetic modifications, including epigenetic reactivation of Nrf2.
Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway - Xuling Su,Xin Jiang, Lingbin Meng, Xiaoming Dong, Yanjun Shen, and Ying Xin - Oxidative Medicine and Cellular Longevity Volume 2018, Article ID 5438179, 10 pages
Activité antibactérienne du sulforaphane et d’autres isothiocyanates
Dans cette étude nous avons tout d'abord effectue un travail de criblage qui a permis de déterminer quelles espèces bactériennes étaient préférentiellement inhibées par le sulforaphane. Nous avons ensuite évalué l'activité in vitro d'autres isothiocyanates sur les espèces bactériennes.
Les isothiocyanates ont fait l'objet de nombreuses évaluations qui ont permis de mettre en évidence, entre autres, leurs propriétés anti carcinogènes ainsi que leur activité antibactérienne. A ce propos, Fahey et coll ont récemment montre que l 'un de ces isothiocyanates, le sulforaphane, protégeait contre la cancérogenèse gastrique induite par le benzo[a]pyrene chez la souris et était de surcroit bactériostatique et bactéricide vis-a-vis de Helicobacter pylori, bactérie impliquée dans les processus d'ulcérogènes et de cancérogenèse gastriques. Le sulforaphane offrirait donc un effet doublement protecteur : non seulement en inhibant les process us de carcinogenèse mais aussi en contribuant à l'éradication de H pylori.
Dans cette étude nous avons tout d'abord effectue un travail de criblage qui a permis de déterminer quelles espèces bactériennes étaient préférentiellement inhibées par le sulforaphane. Nous avons ensuite évalué l'activité in vitro d'autres isothiocyanates sur les espèces bactériennes pour lesquelles les concentrations minimales inhibitrices (CMI) du sulforaphane étaient les plus basses (bactéries appartenant au genre Campylobacter). Enfin, nous avons déterminé les CMI de l'érythromycine et de la ciprofloxacine vis-a-vis des souches de Campylobacter spp. et avons comparé l'efficacité de ces deux antibiotiques habituellement préconises pour le traitement des entérites a Campylobacter spp., à celle du sulforaphane.
Activité antibactérienne du sulforaphane et d'autres isothiocyanates, thèse pour le diplôme d'état de Docteur en Pharmacie, Ludovic Woelffel
Prevention trials - Targeting Nrf2 with Foods Rich in Sulforaphane
Prevention trials of whole foods or simple extracts offer prospects for reducing an expanding global burden of cancer effectively with minimal cost, in contrast to promising isolated phytochemicals or pharmaceuticals. Sulforaphane- or glucoraphanin-rich broccoli sprout extracts provide one avenue towards this end
Abstract : With the properties of efficacy, safety, tolerability, practicability and low cost, foods containing bioactive phytochemicals are gaining significant attention as elements of chemoprevention strategies against cancer. Sulforaphane [1-isothiocyanato-4-(methylsulfinyl)butane], a naturally occurring isothiocyanate produced by cruciferous vegetables such as broccoli, is found to be a highly promising chemoprevention agent against not only variety of cancers such as breast, prostate, colon, skin, lung, stomach or bladder carcinogenesis, but also cardiovascular disease, neurodegenerative diseases, and diabetes. For reasons of experimental exigency, pre-clinical studies have focused principally on sulforaphane itself, while clinical studies have relied on broccoli sprout preparations rich in either sulforaphane or its biogenic precursor, glucoraphanin. Substantive subsequent evaluation of sulforaphane pharmacokinetics and pharmacodynamics has been undertaken using either pure compound or food matrices. Sulforaphane affects multiple targets in cells. One key molecular mechanism of action for sulforaphane entails activation of the Nrf2- Keap1 signaling pathway although other actions contribute to the broad spectrum of efficacy in different animal models. This review summarizes the current status of pre-clinical chemoprevention studies with sulforaphane and highlights the progress and challenges for the application of foods rich in sulforaphane and/or glucoraphanin in the arena of clinical chemoprevention.
Conclusion : Prevention trials of whole foods or simple extracts offer prospects for reducing an expanding global burden of cancer effectively with minimal cost, in contrast to promising isolated phytochemicals or pharmaceuticals. Sulforaphane- or glucoraphanin-rich broccoli sprout extracts provide one avenue towards this end. Clinical trial results demonstrating modulation of exposure (and risk) biomarkers for environmental carcinogens, notably aflatoxins and air pollutants, offer a prospect of impact. A recent placebo-controlled, double-blind randomized trial in which daily oral doses of dietary sulforaphane over 18 weeks demonstrated substantial improvements in markers of autism spectrum disorder further highlights the possible impact on conditions other than cancer. Together with other clinical trial results heralding beneficial actions of drugs known to affect Nrf2 signaling, notably dimethylfumarate as an FDA-approved treatment for multiple sclerosis and bardoxolone methyl for chronic kidney disease, there is optimism that the overall strategies are moving forward. Further refinements in formulation, consistency in bioavailability, development of informative pharmacodynamic biomarkers and broadened demonstrations of efficacy, while maintaining frugality, will be required to enhance the use of food-based approaches to chemoprevention.
Frugal Chemoprevention: Targeting Nrf2 with Foods Rich in Sulforaphane - Li Yang, Dushani L. Palliyaguru, and Thomas W. Kensler - Semin Oncol. 2016 February ; 43(1): 146–153
Targeting oxidative stress in disease
Review of the relationships between oxidative stress, redox signalling and disease, the mechanisms through which oxidative stress can contribute to pathology, how antioxidant defences work...
Abstract : Oxidative stress is a component of many diseases, including atherosclerosis, chronic obstructive pulmonary disease, Alzheimer disease and cancer. Although numerous small molecules evaluated as antioxidants have exhibited therapeutic potential in preclinical studies, clinical trial results have been disappointing. A greater understanding of the mechanisms through which antioxidants act and where and when they are effective may provide a rational approach that leads to greater pharmacological success. Here, we review the relationships between oxidative stress, redox signalling and disease, the mechanisms through which oxidative stress can contribute to pathology, how antioxidant defences work, what limits their effectiveness and how antioxidant defences can be increased through physiological signalling, dietary components and potential pharmaceutical intervention.
Oxidative stress Imbalance between generation of oxidants and the ability to prevent oxidative damage favouring the latter process.
Redox signalling Signal transduction in which oxidants act as second messengers.
Antioxidant defence Prevention or repair of oxidative damage.
Antioxidant enzymes Strictly, enzymes that remove oxidants; broadly, enzymes that contribute to the prevention or repair of oxidative damage. The broader definition is used in this Review.
Targeting oxidative stress in disease: promise and limitations of antioxidant therapy, Henry Jay Forman and Hongqiao Zhang, NATURE REVIEWS, 2021
Sulforaphane reactivates cellular antioxidant defense
Nrf2 and its mediated genes are dysregulated in aging LECs and lenses. Importantly, activation of Nrf2 can be reinforced by treating aged lens cells with SFN. The study also detailed the molecular mechanism that occurs during aging, at least in lens/LECs, i.e.,. Based upon this work, we propose a chemopreventive strategy of using small molecules like SFN to block/delay cataractogenesis or etiopathogenesis in eye lens.
Upon oxidative stress and aging, Nrf2 (NFE2-related factor2) triggers antioxidant defense genes to defends against homeostatic failure. Using human(h) or rat(r) lens epithelial cells (LECs) and aging human lenses, we showed that a progressive increase in oxidative load during aging was linked to a decline in Prdx6 expression. DNA binding experiments using gel-shift and ChIP assays demonstrated a progressive reduction in Nrf2/ARE binding (−357/−349) of Prdx6 promoter. The promoter (−918) with ARE showed a marked reduction in young vs aged hLECs, which was directly correlated to decreased Nrf2/ARE binding. A Nrf2 activator, Sulforaphane (SFN), augmented Prdx6, catalase and GSTπ expression in dose-dependent fashion, and halted Nrf2 dysregulation of these antioxidants. SFN reinforced Nrf2/DNA binding and increased promoter activities by enhancing expression and facilitating Nrf2 translocalization in nucleus. Conversely, promoter mutated at ARE site did not respond to SFN, validating the SFN-mediated restoration of Nrf2/ARE signaling. Furthermore, SFN rescued cells from UVB-induced toxicity in dose-dependent fashion, which was consistent with SFN’s dose-dependent activation of Nrf2/ARE interaction. Importantly, knockdown of Prdx6 revealed that Prdx6 expression was prerequisite for SFN-mediated cytoprotection. Collectively, our results suggest that loss of Prdx6 caused by dysregulation of ARE/Nrf2 can be attenuated through a SFN, to combat diseases associated with aging.
Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2 / ARE / Prdx6 activity during aging and oxidative stress, Kubo et al., 201, SCiENtifiC ReportS, 7: 141307
Sulforaphane Treatment in Alzheimer’s Disease
SFN was observed to improve cognitive function and to protect against Aβ deposition in AD model mice, and the mechanism underlying these effects may be associated with up-regulation of p75 neurotrophin receptor mediated.
Alzheimer’s disease is an irreversible, progressive neurodegenerative disorder. The accumulation of Ab in the brain is thought to play a causative role in the development of cognitive dysfunction in Alzheimer’s disease. The p75 neurotrophin receptor is of great importance to protect against the Ab burden and its expression is regulated by histone acetylation. This study investigated whether the phytochemical sulforaphane, a pan-histone deacetylase inhibitor, up-regulates the p75 neurotrophin receptor expression via affecting histone acetylation in protection against Alzheimer’s disease. We found that sulforaphane ameliorated behavioral cognitive impairments and attenuated brain Ab burden in Alzheimer’s disease model mice. Additionally, sulforaphane reduced the expression of histone deacetylase1, 2, and 3, up-regulated p75 neurotrophin receptor, and increased levels of acetylated histone 3 lysine 9 and acetylated histone 4 lysine 12 in the cerebral cortex of Alzheimer’s diseasemodelmice as well as in Ab-exposed SH-SY5Y cells. Furthermore, silencing of histone deacetylase1 and 3, but not histone deacetylase2, gene expression with small interfering RNA caused up-regulation of p75 neurotrophin receptor in SH-SY5Y cells. In conclusion, this study demonstrates that sulforaphane can ameliorate neurobehavioral deficits and reduce the Ab burden in Alzheimer’s disease model mice, and the mechanism underlying these effects may be associated with up-regulation of p75 neurotrophin receptor mediated, apparently at least in part, via reducing the expression of histone deacetylase1 and 3.
Keywords: Alzheimer’s disease, amyloid-b, sulforaphane, p75 neurotrophin receptor, histone deacetylases
Beneficial Effects of Sulforaphane Treatment in Alzheimer’s Disease May Be Mediated through Reduced HDAC1/3 and Increased P75NTR Expression Jingzhu Zhang, Rui Zhang, Zhipeng Zhan, Xinhui Li, Fuyuan Zhou, Aiping Xing, Congmin Jiang, Yanqiu Chen and Li An, Frontiers in Aging Neuroscience, May 2017, Volume 9, Article 121
Sulforaphane Protects against Cardiovascular Disease
Oxidative stress plays a major role in the pathophysiology of cardiac disorders. Animal and human experiments have identified substantial SFNmediated protection from a range of CVD, including hypertension, atherosclerosis, I/R injury, diabetes, and diabetic complications
Abstract : Cardiovascular disease (CVD) causes an unparalleled proportion of the global burden of disease and will remain themain cause of mortality for the near future. Oxidative stress plays a major role in the pathophysiology of cardiac disorders. Several studies have highlighted the cardinal role played by the overproduction of reactive oxygen or nitrogen species in the pathogenesis of ischemic myocardial damage and consequent cardiac dysfunction. Isothiocyanates (ITC) are sulfur-containing compounds that are broadly distributed among cruciferous vegetables. Sulforaphane (SFN) is an ITC shown to possess anticancer activities by both in vivo and epidemiological studies. Recent data have indicated that the beneficial effects of SFN in CVD are due to its antioxidant and antiinflammatory properties. SFN activates NF-E2-related factor 2 (Nrf2), a basic leucine zipper transcription factor that serves as a defense mechanism against oxidative stress and electrophilic toxicants by inducing more than a hundred cytoprotective proteins, including antioxidants and phase II detoxifying enzymes. This review will summarize the evidence fromclinical studies and animal experiments relating to the potential mechanisms by which SFN modulates Nrf2 activation and protects against CVD.
Conclusion : Oxidative stress plays a major role in the pathophysiology of cardiac disorders. SFN found in cruciferous vegetables is an indirect antioxidant that can activate Nrf2 and its downstream target genes to induce antioxidant effects. Animal and human experiments have identified substantial SFNmediated protection from a range of CVD, including hypertension, atherosclerosis, I/R injury, diabetes, and diabetic complications (Table 1).The findings presented in this review indicate that SFN, a phytochemical isolated from extracts of an edible plant with a presumed low level of toxicity, protects against CVD. SFN could therefore contribute to the prevention of CVD.
Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation Yang Bai, Xiaolu Wang, Song Zhao, Chunye Ma, Jiuwei Cui, and Yang Zheng, Oxidative Medicine and Cellular Longevity, Volume 2015, Article ID 407580, 13 pages
Neuroprotective mechanisms and effects of sulforaphane
SFN is a powerful antioxidant and anti‑inflammatory phytochemical with great promise in its ability to protect the nervous system from many diseases and toxins and reduce the symptomatic burden of multiple pervasive diseases
Abstract: Sulforaphane (SFN) is a phytochemical found in cruciferous vegetables. It has been shown to have many protective effects against many diseases, including multiple types of cancer. SFN is a potent activator of the nuclear factor erythroid 2‑related factor 2 (Nrf2) antioxidant response element (ARE) genetic pathway. Upregulation of Nrf2‑ARE increases the availability of multiple antioxidants. A substantial amount of preclinical research regarding the ability of SFN to protect the nervous system from many diseases and toxins has been done, but only a few small human trials have been completed. Preclinical data suggest that SFN protects the nervous system through multiple mechanisms and may help reduce the risk of many diseases and reduce the burden of symptoms in existing conditions. This review focuses on the literature regarding the protective effects of SFN on the nervous system. A discussion of neuroprotective mechanisms is followed by a discussion of the protective effects elicited by SFN administration in a multitude of neurological diseases and toxin exposures. SFN is a promising neuroprotective phytochemical which needs further human trials to evaluate its efficacy in preventing and decreasing the burden of many neurological diseases.
Conclusion and Perspective : SFN is a powerful antioxidant and anti‑inflammatory phytochemical with great promise in its ability to protect the nervous system from many diseases and toxins and reduce the symptomatic burden of multiple pervasive diseases. Research regarding long‑term use in humans and disease outcomes will be important to determine its clinical utility. SFN, found naturally in high concentrations in broccoli sprouts, is a powerful example of how important food is to our health, and we must remember that while simple things like broccoli do not seem as powerful as human‑made pharmaceuticals, they can truly be as or more important. The area of phytochemical use in prevention and damage reduction of neurological diseases is blossoming and may well be an important next step in reducing the risk of the many diseases we have assumed inevitable or incurable.
Keywords: Antioxidant, autism spectrum disorder, broccoli sprouts, epilepsy, isothiocyanate, neurodegenerative disease, nuclear factor erythroid 2‑related factor 2, phytochemical, schizophrenia
The neuroprotective mechanisms and effects of sulforaphane, Eric A Klomparens, Yuchuan Ding, 2019 Brain Circulation
Sulforaphane, a naturally occurring Nrf2 activator
Pretreatment with sulforaphane, a naturally occurring Nrf2 activator, prevented depression-like phenotype in mice after inflammation, or chronic social defeat stress. It is, therefore, possible that dietary intake of cruciferous vegetables including glucoraphanin (or SFN) may prevent or minimize relapse from remission, induced by stress and/or inflammation in depressed patients.
Abstract : Depression is one of the most common mood disorders with a high rate of relapse. Accumulating evidence suggests that the transcription factor Kelch-like erythroid cell-derived protein with CNC homology (ECH)-associated protein 1 (Keap1)-Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) system plays a key role in inflammation which is involved in depression. Preclinical studies demonstrated that the protein expressions of Keap1 and Nrf2 in the prefrontal cortex (PFC), CA3 and dentate gyrus (DG) of hippocampus in mice with depression-like phenotype were lower than control mice. In the learned helplessness paradigm, the protein levels of Keap1 and Nrf2 in the PFC and DG of hippocampus from rats with depression-like phenotype were also lower than control and resilient rats. Furthermore, rodents with depression-like phenotype have higher levels of pro-inflammatory cytokines. Interestingly, Nrf2 knock-out (KO) mice exhibit depression-like phenotype, and higher serum levels of pro-inflammatory cytokines compared with wild-type mice. Furthermore, Nrf2 KO mice have lower expression of brain-derived neurotrophic factor (BDNF) in the PFC, and CA3 and DG of hippocampus compared to wild-type mice. 7,8-Dihydroxyflavone, a TrkB agonist, showed antidepressant effects in Nrf2 KO mice, by stimulating BDNF-TrkB in the PFC, CA3, and DG. Pretreatment with sulforaphane, a naturally occurring Nrf2 activator, prevented depression-like phenotype in mice after inflammation, or chronic social defeat stress. Interestingly, dietary intake of 0.1% glucoraphanin (a precursor of sulforaphane) containing food during juvenile and adolescent stages of mice could prevent depression-like phenotype in adulthood after chronic social defeat stress. Moreover, the protein expressions of Keap1 and Nrf2 in the parietal cortex from major depressive disorder and bipolar disorder were lower than controls. These findings suggest that Keap1-Nrf2 system plays a key role in the stress resilience which is involved in the pathophysiology of mood disorders. It is, therefore, possible that dietary intake of cruciferous vegetables including glucoraphanin (or SFN) may prevent or minimize relapse from remission, induced by stress and/or inflammation in depressed patients. In the review, the author would like to discuss the role of Keap1-Nrf2 system in mood disorders. In the review, the author would like to discuss the role of Keap1-Nrf2 system in mood disorders.
Keywords: brain-derived neurotrophic factor, glucoraphanin, keap1, Nrf2, nutrition, stress resilience, sulforaphane, TrkB
Essential Role of Keap1-Nrf2 Signaling in Mood Disorders: Overview and Future Perspective, Kenji Hashimoto, Frontiers in Pharmacology, October 2018, Volume 9, Article 1182
Sulforaphane and metabolite, children with Autism Spectrum Disorder
Improvements in sociability and communication were observed, as well as irritability, stereotypy, hyperactivity, and inappropriate speech on the ABC with a non-randomized analysis of the length of exposure. SF also had significant positive effects on oxidative stress, cytoprotective markers and cytokines, as well as mitochondrial function.
Background: Sulforaphane (SF), an isothiocyanate in broccoli, has potential benefits relevant to autism spectrum disorder (ASD) through its effects on several metabolic and immunologic pathways. Previous clinical trials of oral SF demonstrated positive clinical effects on behavior in young men and changes in urinary metabolomics in children with ASD.
Methods: We conducted a 15-week randomized parallel double-blind placebo-controlled clinical trial with 15-week open-label treatment and 6-week no-treatment extensions in 57 children, ages 3–12 years, with ASD over 36 weeks. Twenty-eight were assigned SF and 29 received placebo (PL). Clinical effects, safety and tolerability of SF were measured as were biomarkers to elucidate mechanisms of action of SF in ASD.
Results: Data from 22 children taking SF and 23 on PL were analyzed. Treatment effects on the primary outcome measure, the Ohio Autism Clinical Impressions Scale (OACIS), in the general level of autism were not significant between SF and PL groups at 7 and 15 weeks. The effect sizes on the OACIS were non-statistically significant but positive, suggesting a possible trend toward greater improvement in those on treatment with SF (Cohen’s d 0.21; 95% CI − 0.46, 0.88 and 0.10; 95% CI − 0.52, 0.72, respectively). Both groups improved in all subscales when on SF during the open-label phase. Caregiver ratings on secondary outcome measures improved significantly on the Aberrant Behavior Checklist (ABC) at 15 weeks (Cohen’s d − 0.96; 95% CI − 1.73, − 0.15), but not on the Social Responsiveness Scale-2 (SRS-2). Ratings on the ABC and SRS-2 improved with a non-randomized analysis of the length of exposure to SF, compared to the pre-treatment baseline (p max 0.001). There were significant changes with SF compared to PL in biomarkers of glutathione redox status, mitochondrial respiration, inflammatory markers and heat shock proteins. Clinical laboratory studies confirmed product safety. SF was very well tolerated and side effects of treatment, none serious, included rare insomnia, irritability and intolerance of the taste and smell.
Limitations: The sample size was limited to 45 children with ASD and we did not impute missing data. We were unable to document significant changes in clinical assessments during clinical visits in those taking SF compared to PL. The clinical results were confounded by placebo effects during the open-label phase.
Conclusions We found that SF was safe, but based upon the OACIS, our primary clinical outcome measure, its effects were not significant. Effect size estimates showed small improvements with SF, though not significant, for the general level of autism on the OACIS-I scale. There was significant improvement on one of the secondary measures (ABC) but not on the SRS-2. Improvements in sociability and communication were observed on the SRS-2, as well as irritability, stereotypy, hyperactivity, and inappropriate speech on the ABC with a non-randomized analysis of the length of exposure. SF also had significant positive effects on oxidative stress, cytoprotective markers and cytokines, as well as mitochondrial function. These were promising findings that require further investigation of both the clinical effects and mechanisms of action of SF.
Randomized controlled trial of sulforaphane and metabolite discovery in children with Autism Spectrum Disorder, Andrew W. Zimmerman, Kanwaljit Singh, Susan L. Connors, Hua Liu, Anita A. Panjwani, Li‑Ching Lee, Eileen Diggins, Ann Foley, Stepan Melnyk, Indrapal N. Singh, S. Jill James, Richard E. Frye and Jed W. Fahey, Molecular Autism (2021) 12:38
Modulating NRF2 in disease: Timing is everything
There are over 250 currently identified NRF2 target genes involved in a multitude of cellular processes. Interestingly, a number of NRF2 activators, including sulforaphane (SF) have all been shown to suppress NF-KB signaling
Abstract : The transcription factor nuclear factor erythroid-2 (NF-E2)-related factor 2 (NRF2) is a central regulator of redox, metabolic, and protein homeostasis that intersects with many other signaling cascades. While the understanding of the complex nature of NRF2 signaling continues to grow, there is only one therapeutic targeting NRF2 for clinical use, dimethyl fumarate, used for the treatment of multiple sclerosis. The discovery of new therapies is confounded by the fact that NRF2 levels vary significantly depending on physiological and pathological context. Thus, properly timed and targeted manipulation of the NRF2 pathway is critical in creating effective therapeutic regimens. In this review, we summarize the regulation and downstream targets of NRF2. Furthermore, we discuss the role of NRF2 in cancer, neurodegeneration, and diabetes, as well as cardiovascular, kidney, and liver disease, with a special emphasis on NRF2-based therapeutics, including those that have made it into clinical trials.
Keywords : NRF2; KEAP1; therapeutics; cancer; disease; clinical trials; sulforaphane
Modulating NRF2 in disease: Timing is everything Matthew Dodson, Montserrat Rojo de la Vega1 Aram B. Cholanians, Cody J. Schmidlin, Eli Chapman, and Donna D. Zhang, Annu Rev Pharmacol Toxicol. 2019 January 06; 59: 555–575
Diversity and distribution of glucosinolates andisothiocyanates
Glucosinolates are present in sixteen families including a large number of edible species, primarily the family Brassicaceae
Abstract : Glucosinolates (-thioglucoside-N-hydroxysulfates), the precursors of isothiocyanates, are present in sixteen families of dicoty- ledonous angiosperms including a large number of edible species. At least 120 dierent glucosinolates have been identi®ed in these plants, although closely related taxonomic groups typically contain only a small number of such compounds. Glucosinolates and/or their breakdown products have long been known for their fungicidal, bacteriocidal, nematocidal and allelopathic properties and have recently attracted intense research interest because of their cancer chemoprotective attributes. Numerous reviews have addressed the occurrence of glucosinolates in vegetables, primarily the family Brassicaceae (syn. Cruciferae; including Brassica spp and Raphanus spp). The major focus of much previous research has been on the negative aspects of these compounds because of the prevalence of certain ``antinutritional'' or goitrogenic glucosinolates in the protein-rich defatted meal from widely grown oilseed crops and in some domesticated vegetable crops. There is, however, an opposite and positive side of this picture represented by the therapeutic and prophylactic properties of other ``nutritional'' or ``functional'' glucosinolates. This review addresses the complex array of these biologically active and chemically diverse compounds many of which have been identi®ed during the past three dec- ades in other families. In addition to the Brassica vegetables, these glucosinolates have been found in hundreds of species, many of which are edible or could provide substantial quantities of glucosinolates for isolation, for biological evaluation, and potential application as chemoprotective or other dietary or pharmacological agents. Keywords: Cancer; Chemoprotection; Edible plants; Functional food; Myrosinase; Crucifers
The chemical diversity and distribution of glucosinolates and isothiocyanates among plants - Jed W. Fahey, Amy T. Zalcmann, Paul Talalay - J.W. Fahey et al. / Phytochemistry 56 (2001) 5-51
Sulforaphane in the Prevention and Treatment of Chronic Disease
This review has explored the issues associated with the development of a nutraceutical supplement with significant ability to beneficially influence many of the upstream processes associated with core cellular defences; SFN emerges as a potential candidate of this class.
Abstract : A growing awareness of the mechanisms by which phytochemicals can influence upstream endogenous cellular defence processes has led to intensified research into their potential relevance in the prevention and treatment of disease. Pharmaceutical medicine has historically looked to plants as sources of the starting materials for drug development; however, the focus of nutraceutical medicine is to retain the plant bioactive in as close to its native state as possible. As a consequence, the potency of a nutraceutical concentrate or an extract may be lower than required for significant gene expression. The molecular structure of bioactive phytochemicals to a large extent determines the molecule’s bioavailability. Polyphenols are abundant in dietary phytochemicals, and extensive in vitro research has established many of the signalling mechanisms involved in favourably modulating human biochemical pathways. Such pathways are associated with core processes such as redox modulation and immune modulation for infection control and for downregulating the synthesis of inflammatory cytokines. Although the relationship between oxidative stress and chronic disease continues to be affirmed, direct-acting antioxidants such as vitamins A, C, and E, beta-carotene, and others have not yielded the expected preventive or therapeutic responses, even though several large meta-analyses have sought to evaluate the potential benefit of such supplements. Because polyphenols exhibit poor bioavailability, few of their impressive in vitro findings have been replicated in vivo. SFN, an aliphatic isothiocyanate, emerges as a phytochemical with comparatively high bioavailability. A number of clinical trials have demonstrated its ability to produce favourable outcomes in conditions for which there are few satisfactory pharmaceutical solutions, foreshadowing the potential for SFN as a clinically relevant nutraceutical. Although myrosinase-inert broccoli sprout extracts are widely available, there now exist myrosinase-active broccoli sprout supplements that yield sufficient SFN to match the doses used in clinical trials.
Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease, Christine A. Houghton - Oxidative Medicine and Cellular Longevity Volume 2019, Article ID 2716870, 27 pages
Selective toxicity of Sulforaphane
A selective toxicity of SFR to cancer cells has been demonstrated in different experimental models. As an example, SFR induces cytotoxic and cytostatic effects in different prostate cancer cell lines, but not in their normal counterpart
Abstract : According to recent estimates, cancer continues to remain the second leading cause of death and is becoming the leading one in old age. Failure and high systemic toxicity of conventional cancer therapies have accelerated the identification and development of innovative preventive as well as therapeutic strategies to contrast cancer-associated morbidity and mortality. In recent years, increasing body of in vitro and in vivo studies has underscored the cancer preventive and therapeutic efficacy of the isothiocyanate sulforaphane. In this review article, we highlight that sulforaphane cytotoxicity derives from complex, concurring, and multiple mechanisms, among which the generation of reactive oxygen species has been identified as playing a central role in promoting apoptosis and autophagy of target cells. We also discuss the site and the mechanism of reactive oxygen species’ formation by sulforaphane, the toxicological relevance of sulforaphane-formed reactive oxygen species, and the death pathways triggered by sulforaphane-derived reactive oxygen species.
Conclusion : Although further studies are needed to clarify the relative importance of ROS in SFR toxicity, the effects which tie to ROS generation are definitely important for the ITC’s cytotoxic activity and may represent the bases for its rationale exploitation in cancer therapy as a single agent or in association with other antineoplastic agents or drugs to potentiate their anticancer efficacy.
Cytotoxic and Antitumor Activity of Sulforaphane: The Role of Reactive Oxygen Species - Piero Sestili and Carmela Fimognari, Hindawi Publishing Corporation, BioMed Research International
Sulforaphane inhibits the expression of interleukin-6 and interleukin-8
The control of the cytokine storm is one of the major issues in the management of COVID-19 patients. This study suggests that SFN can be employed in protocols useful to control hyperinflammatory state associated with SARS-CoV-2 infection
Background: A key clinical feature of COVID-19 is a deep inflammatory state known as “cytokine storm” and characterized by high expression of several cytokines, chemokines and growth factors, including IL-6 and IL-8. A direct consequence of this inflammatory state in the lungs is the Acute Respiratory Distress Syndrome (ARDS), frequently observed in severe COVID-19 patients. The "cytokine storm" is associated with severe forms of COVID- 19 and poor prognosis for COVID-19 patients. Sulforaphane (SFN), one of the main components of Brassica oleraceae L. (Brassicaceae or Cruciferae), is known to possess anti-inflammatory effects in tissues from several organs, among which joints, kidneys and lungs.
Purpose: The objective of the present study was to determine whether SFN is able to inhibit IL-6 and IL-8, two key molecules involved in the COVID-19 "cytokine storm".
Methods: The effects of SFN were studied in vitro on bronchial epithelial IB3-1 cells exposed to the SARS-CoV-2 Spike protein (S-protein). The anti-inflammatory activity of SFN on IL-6 and IL-8 expression has been evaluated by RT-qPCR and Bio-Plex analysis.
Results: In our study SFN inhibits, in cultured IB3-1 bronchial cells, the gene expression of IL-6 and IL-8 induced by the S-protein of SARS-CoV-2. This represents the proof-of-principle that SFN may modulate the release of some key proteins of the COVID-19 "cytokine storm".
Conclusion: The control of the cytokine storm is one of the major issues in the management of COVID-19 patients. Our study suggests that SFN can be employed in protocols useful to control hyperinflammatory state associated with SARS-CoV-2 infection.
Keywords: COVID-19 Spike sulforaphane biomarkers inflammation nutraceuticals
Sulforaphane inhibits the expression of interleukin-6 and interleukin-8 induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 Spike protein Jessica Gasparello et al., Phytomedicine 87 (2021) 153583
Can Activation of NRF2 Be a Strategy against COVID-19?
The available information on pharmacokinetics, pharmacodynamics, safety, and efficacy for the NRF2 activators sulforaphane and bardoxolone methyl (currently in advanced clinical trials for other disease indications) in humans makes them excellent candidates for testing in randomized clinical trials in COVID-19.
Abstract : Acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 is largely the result of a dysregulated host response, followed by damage to alveolar cells and lung fibrosis. Exacerbated proinflammatory cytokines release (cytokine storm) and loss of T lymphocytes (leukopenia) characterize the most aggressive presentation. We propose that a multifaceted anti-inflammatory strategy based on pharmacological activation of nuclear factor erythroid 2 p45-related factor 2 (NRF2) can be deployed against the virus. The strategy provides robust cytoprotection by restoring redox and protein homeostasis, promoting resolution of inflammation, and facilitating repair. NRF2 activators such as sulforaphane and bardoxolone methyl are already in clinical trials. The safety and efficacy information of these modulators in humans, together with their welldocumented cytoprotective and anti-inflammatory effects in preclinical models, highlight the potential of this armamentarium for deployment to the battlefield against COVID-19.
Highlights The host inflammatory response is a crucial determinant of disease outcome and correlates with disease severity in SARS-CoV-2-induced infection, for which there is no treatment to date. Activation of transcription factor nuclear factor erythroid 2 p45-related factor 2 (NRF2) promotes resolution of inflammation and, in parallel, restores cellular redox and protein homeostasis, and facilitates tissue repair. NRF2 can be activated by pharmacological inducers that target Kelch-like ECH-associated protein 1 (KEAP1), the principal negative regulator of NRF2. The available information on pharmacokinetics, pharmacodynamics, safety, and efficacy for the NRF2 activators sulforaphane and bardoxolone methyl (currently in advanced clinical trials for other disease indications) in humans makes them excellent candidates for testing in randomized clinical trials in COVID-19.
Can Activation of NRF2 Be a Strategy against COVID-19?, Antonio Cuadrado, Trends in Pharmacological Sciences, September 2020, Vol. 41, No. 9
Variation of Glucosinolates in Vegetable Crops of Brassica oleracea
The diversity in glucosinolate levels reported in this and other studies suggests that the potential health benefits from crucifer vegetables are greatly dependent on the accession selected. It's so very improtant to get the correct source. The wide range of variability in glucosinolates among and within the five Brassica groups that were evaluated in this study offer an important information base for developing accessions with enhanced health benefits.
Abstracts : Glucosinolates were evaluated in 5 groups and 65 accessions of Brassica oleracea (50 broccoli, 4 Brussels sprouts, 6 cabbage, 3 cauliflower, and 2 kale) grown under uniform cultural conditions. Glucosinolates and their concentrations varied among the different groups and within each group. The predominant glucosinolates in broccoli were 4-methylsulfinylbutyl glucosinolate (glucoraphanin), 3-butenyl glucosinolate (gluconapin), and 3-indolylmethyl glucosinoate (glucobrassicin). Glucoraphanin concentration in broccoli ranged from 0.8 ímol g-1 DW in EV6-1 to 21.7 ímol g-1 DW in Brigadier. Concentrations of the other glucosinolates in broccoli varied similarly over a wide range. In Brussels sprouts, cabbage, cauliflower, and kale, the predominant glucosinolates were sinigrin (8.9, 7.8, 9.3, and 10.4 ímol g-1 DW, respectively) and glucobrassicin (3.2, 0.9, 1.3, and 1.2 ímol g-1 DW, respectively). Brussels sprouts also had significant amounts of gluconapin (6.9 ímol g-1 DW). Wide variations in glucosinolate content among genotypes suggest differences in their health-promoting properties and the opportunity for enhancement of their levels through genetic manipulation.
Variation of Glucosinolates in Vegetable Crops of Brassica oleracea - Mosbah M. Kushad, Allan F. Brown, Anne C. Kurilich, John A. Juvik, Barbara P. Klein, Mathew A. Wallig, and Elizabeth H. Jeffery - J. Agric. Food Chem. 1999
Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses
SFN treatment diminished immune cell activation in the lungs, including significantly lower recruitment of myeloid cells and a reduction in T cell activation and cytokine production
Abstract Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has incited a global health crisis. Currently, there are no orally available medications for prophylaxis for those exposed to SARS-CoV-2 and limited therapeutic options for those who develop COVID-19. We evaluated the antiviral activity of sulforaphane (SFN), a naturally occurring, orally available, well-tolerated, nutritional supplement present in high concentrations in cruciferous vegetables with limited side effects. SFN inhibited in vitro replication of four strains of SARS-CoV-2 as well as that of the seasonal coronavirus HCoVOC43. Further, SFN and remdesivir interacted synergistically to inhibit coronavirus infection in vitro. Prophylactic administration of SFN to K18-hACE2 mice prior to intranasal SARS-CoV-2 infection significantly decreased the viral load in the lungs and upper respiratory tract and reduced lung injury and pulmonary pathology compared to untreated infected mice. SFN treatment diminished immune cell activation in the lungs, including significantly lower recruitment of myeloid cells and a reduction in T cell activation and cytokine production. Our results suggest that SFN is a promising treatment for prevention of coronavirus infection or treatment of early disease.
Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses Alvaro A. Ordonez et al., March 25, 2021.
broccoli and glucoraphanin in COVID-19 : three experimental clinical cases
These experimental clinical cases represent a proof-of-concept confirming the hypothesis that Nrf2- interacting nutrients are effective in COVID-19.
ABSTRACT COVID-19 is described in a clinical case involving a patient who proposed the hypothesis that Nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-interacting nutrients may help to prevent severe COVID-19 symptoms. Capsules of broccoli seeds containing glucoraphanin were being taken before the onset of SARS-CoV-2 infection and were continued daily for over a month after the first COVID-19 symptoms. They were found to reduce many of the symptoms rapidly and for a duration of 6–12 h by repeated dosing. When the patient was stable but still suffering from cough and nasal obstruction when not taking the broccoli capsules, a double-blind induced cough challenge confirmed the speed of onset of the capsules (less than 10 min). A second clinical case with lower broccoli doses carried out during the cytokine storm confirmed the clinical benefits already observed. A third clinical case showed similar effects at the onset of symptoms. In the first clinical trial, we used a dose of under 600 mmol per day of glucoraphanin. However, such a high dose may induce pharmacologic effects that require careful examination before the performance of any study. It is likely that the fast onset of action is mediated through the TRPA1 channel. These experimental clinical cases represent a proof-of-concept confirming the hypothesis that Nrf2- interacting nutrients are effective in COVID-19. However, this cannot be used in practice before the availability of further safety data, and confirmation is necessary through proper trials on efficacy and safety
Keywords: COVID-19, Nrf2, Broccoli, Cough challenge, TRPA1, TRPV1
Efficacy of broccoli and glucoraphanin in COVID-19: From hypothesis to proof-ofconcept with three experimental clinical cases Jean Bousquet et al, World Allergy Organization Journal (2021)
Efficacité de Sulfodyne® pour la prise en charge du syndrome prémenstruel : une nouvelle étude en situation réelle
Une étude en conditions de vie réelles sur 50 femmes confirme l'efficacité de SULFODYNE®, l’unique et authentique extrait de sulforaphane naturel et actif pour la gestion du syndrome prémenstruel.
Effectiveness of Sulfodyne® for the management of premenstrual syndrome: a new real-life study
A real-life study on 50 women confirms the effectiveness of SULFODYNE®, the only extract with authentic, natural, active sulforaphane for the management of premenstrual syndrome.
Suylfodyne against viral infection
A preclinical study confirms multi-target mode of action of SULFODYNE® against viral infection.
BECARRE Natural invites you to join us at Vitafoods Europe 2023, 09-11 May in Palexpo, Geneva
As every year, you will be welcome to meet us during Vitafoods 2023. Our wide range of standardized and objectivized plant extracts will be on display at stand J170, Pavillon France.
Platinium Sponsor NBD 2022 and 10th years anniversary
As the main Sponsor, Becarre Natural is waiting for you at Nutriform Business Days, 3 days of sharing and discussions, inspiring conferences and round tables dedicated to international news on the dietary supplements market, scientific and marketing solutions to develop your business.
Sulfodyne® - Prefer the bioactive sulforaphane from broccoli rather than its precursor
Becarre Natural represents Sulfodyne®, the unique extract of stabilized broccoli providing the active sulforaphane.
Newsletter Sept. 2021 - Française
Une sélection rapide des toutes dernières informations sur nos études et nos extraits de plantes standardisés et objectivés, pour la nutrition et la cosmétique.
Newsletter Sept. 2021 - English
A quick selection of the very latest information on our standardized and objectivized plant extracts, and their studies, for nutrition and cosmetics.
Are you interested in our solutions and want to know more?
Becarre Natural represents, distributes and develops natural actives extracts from plants for nutrition health and cosmeceutical.
Contact us